LINEA
ACCELETRIAL
PROJECT
OVERVIEW
⇀ LINEA System wanted to fully automatize their clinical trials process by creating a product that's easy enough to use by all parts involved.
⇀ The main goal was to provide pharma with a tool to speed up the site identification and approval process, in order to accelerate site start-up and patient enrollment.
LET'S TALK
ABOUT IT
01. DEVELOPMENT TEAM
I was acting as the Product Designer on a team of 6 (DEV, Q&A, PM), working closely with my team and with the client’s team, on a daily basis.
We all collaborated through tools like Slack, Jira, Invision, Hangouts, Zoom.
As for role specifics, I took care of the initial documentation & research, early concepts & designs, prototypes, and I also made sure that everything was implemented properly by closely collaborating with the developers.

02. PAIN POINTS, FEATURES & PROJECT TIMELINE
Pain points wise, LINEA System was conducting clinical trials using outdated systems, which relied way too much on manual procedures. And because of this, delays and human errors were pretty common. So this needed to be solved through automation, through a new & modern web platform.
A few of the features that needed to be developed included:
⇀ central repository to drive site identification and store research site data
⇀ access to multiple team members involved in site launch process
⇀ data integration/migration
⇀ interface with existing CTMS applications or other tracking systems
⇀ data analytics to identify, quantify and rank sites
⇀ automated reminders based on pre-set benchmarks for each step
Timeline wise, we worked and delivered the project in about 8-9 months, containing all the required features.
03. INITIAL DOCUMENTATION & RESEARCH
We had an initial kick-off meeting with the founders, where they gave us a general overview of the project. We later received a handful of documents containing business requirements, product goals, success metrics, and flows of their current process.
Me and my team, we went through all the received documents to make sure we are all on the same page, by understanding what we need to achieve, and how.
Throughout this documentation period I was taking notes and thinking on ways of designing the product. I was also drawing possible outcomes and flows in order to set up the foundation, and have these be a discussion starter.
I wasn't able to study similar products, due to the closed nature of this field. So because of that we relied mainly in closely collaborating and discussing with the founders. They were really helpful and available, and so we had countless meetings, discussing all the intricacies. While the initial documents covered a lot of things, at a macro-level, we still had to cover certain things at a micro-level, which we managed to cover and understand thanks to the founders.
04. INTERVIEWS
Based on the initial documentation and research I managed to get a real feel of the overall direction, where LINEA wanted to go with this product, and which were the biggest issues they wanted to solve.
However, I also wanted to find out what a good product would look like for all the other parties involved, pharma teams & sites. Basically what they like and don't like when using a product like this, and I was also curious to find out if there are any other features that are missing from similar products, features they would find helpful, and would make things easier for them.
I didn't personally conduct these interviews, but my questions were passed along to the right people, and then I have received the answers, which were quite helpful in molding the ideal product.
05. USER STORIES & USE CASES
Based on all the data I retrieved throughout the initial documentation and research period, I managed to create user stories, which were helpful in achieving maximum flow coverage. I was also able to identify several edge cases which were worked out with the founders in the most ideal way possible.
06. THE FUN PART - DESIGNS
After going through the initial steps of documentation & research it was time to put things together through designs and prototypes, which we also used for user testing.
There was no branding really set in for the product, the client came in only with the logo. Other than that, it was up to me.
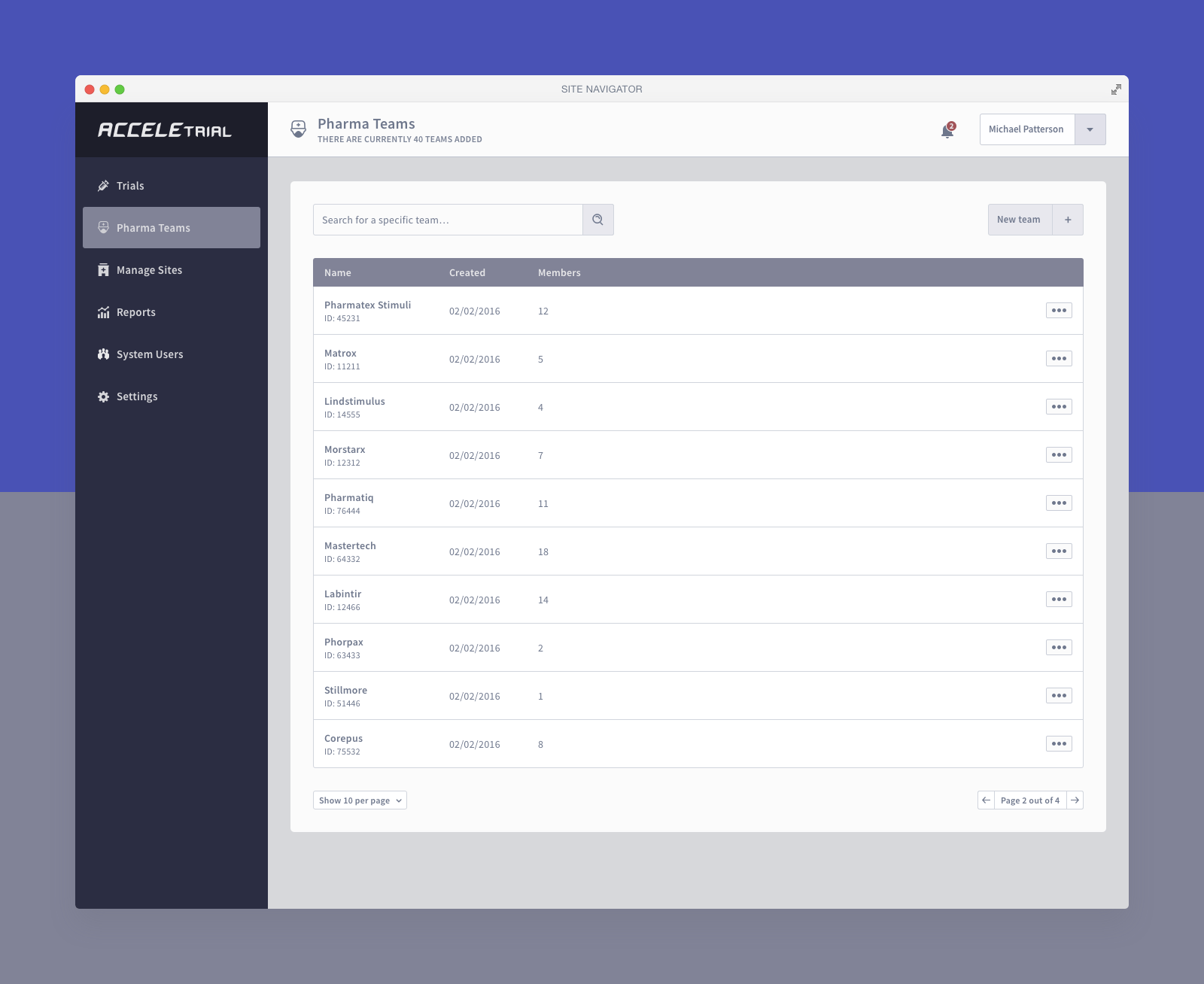
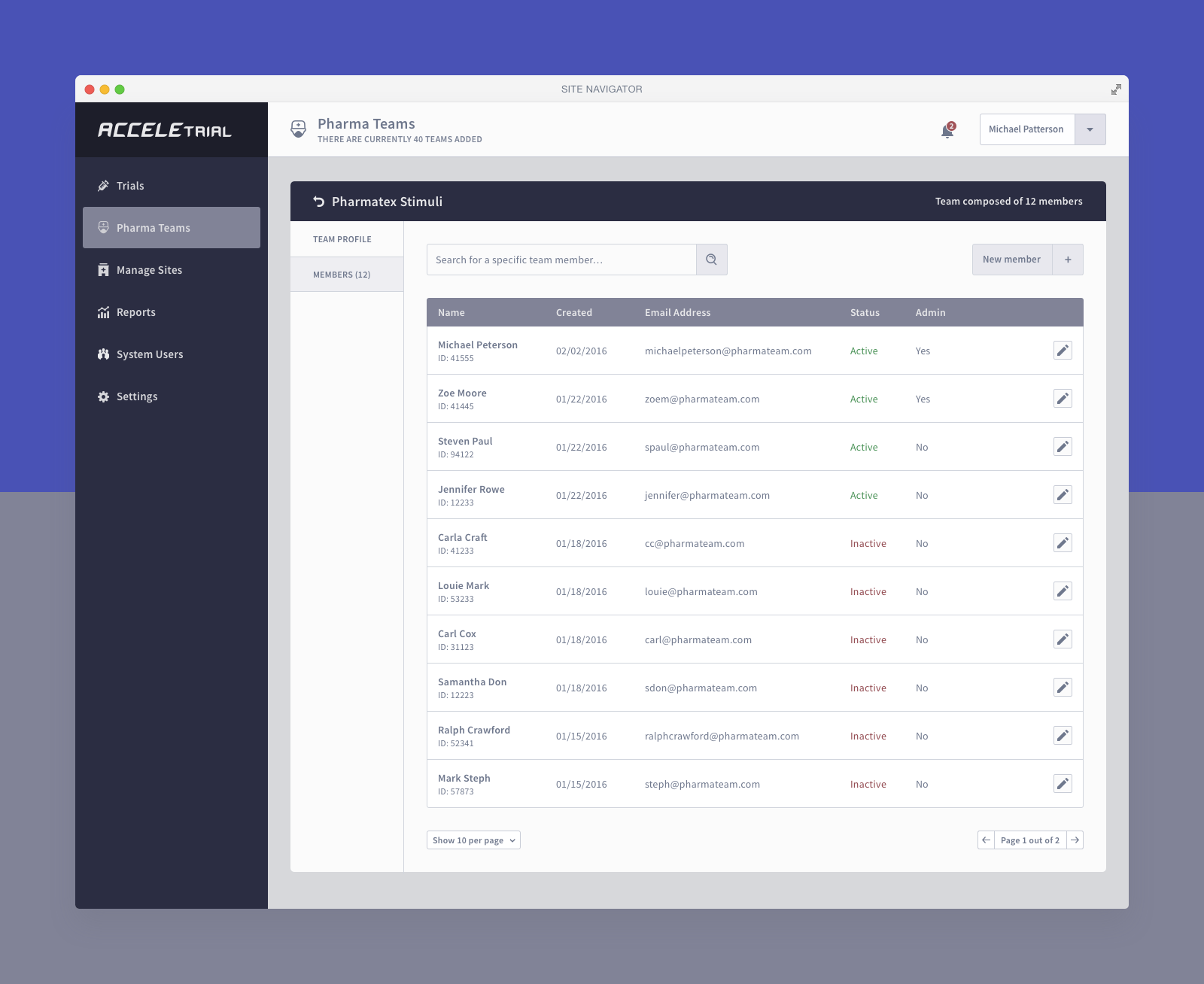
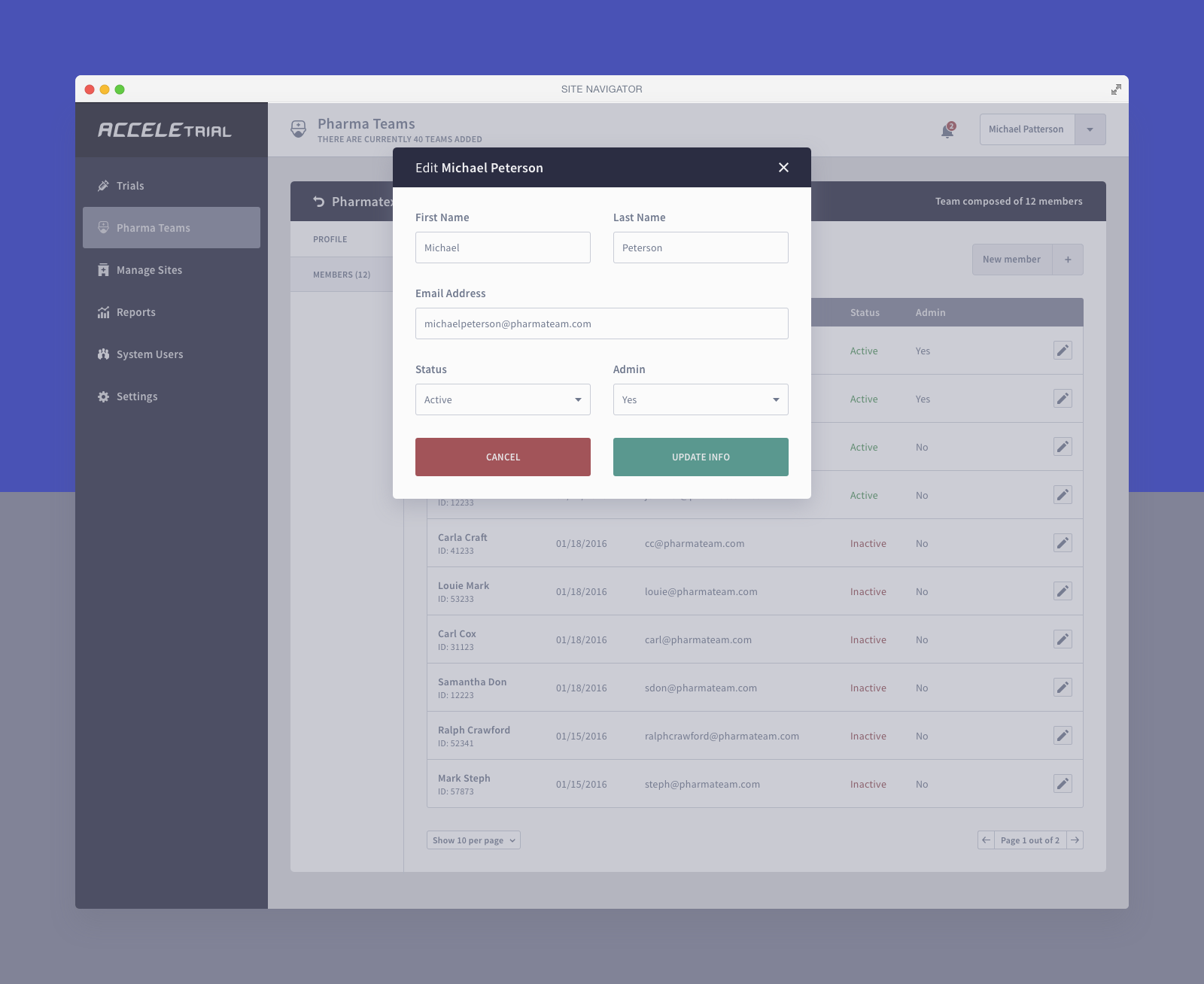
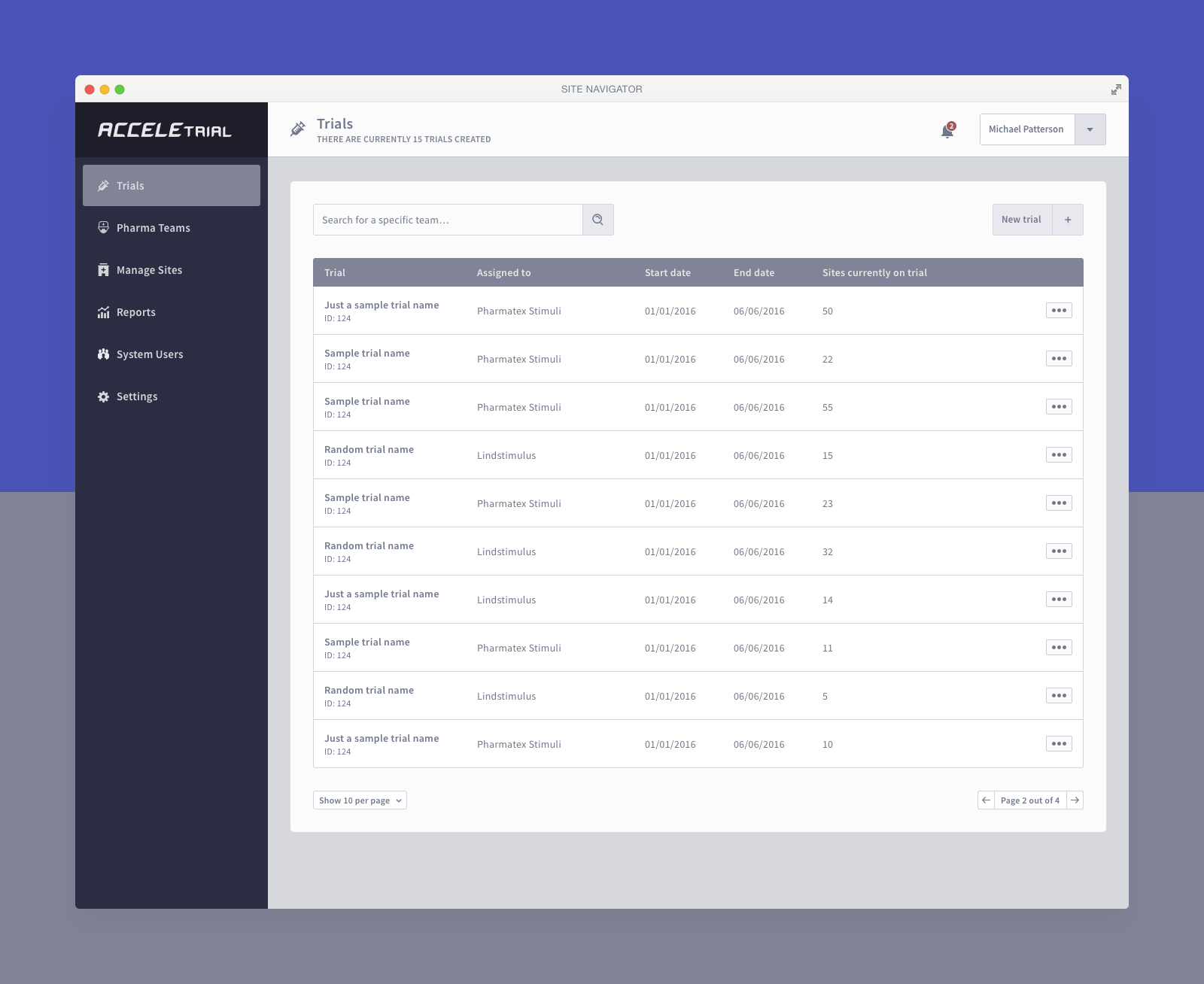
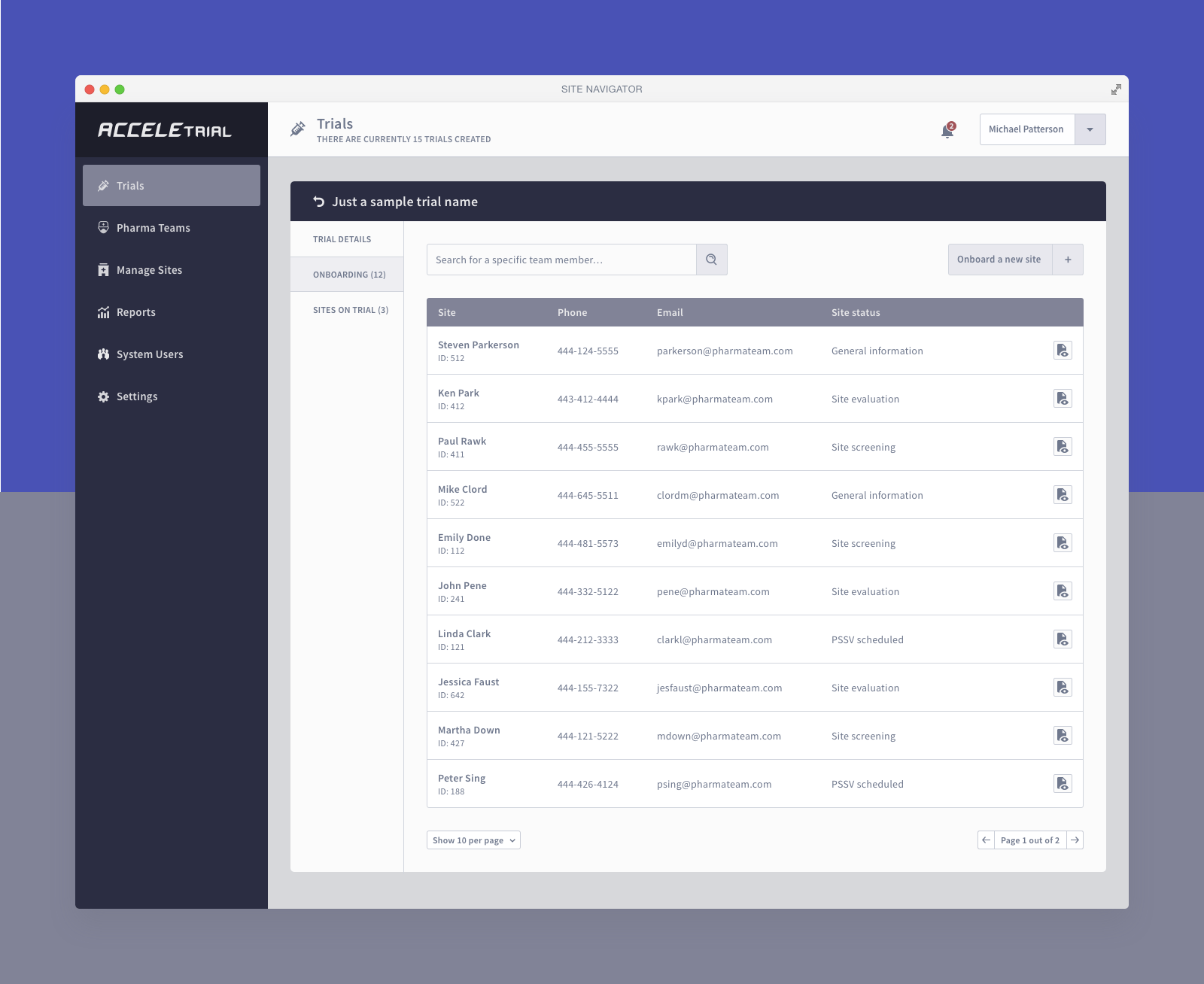
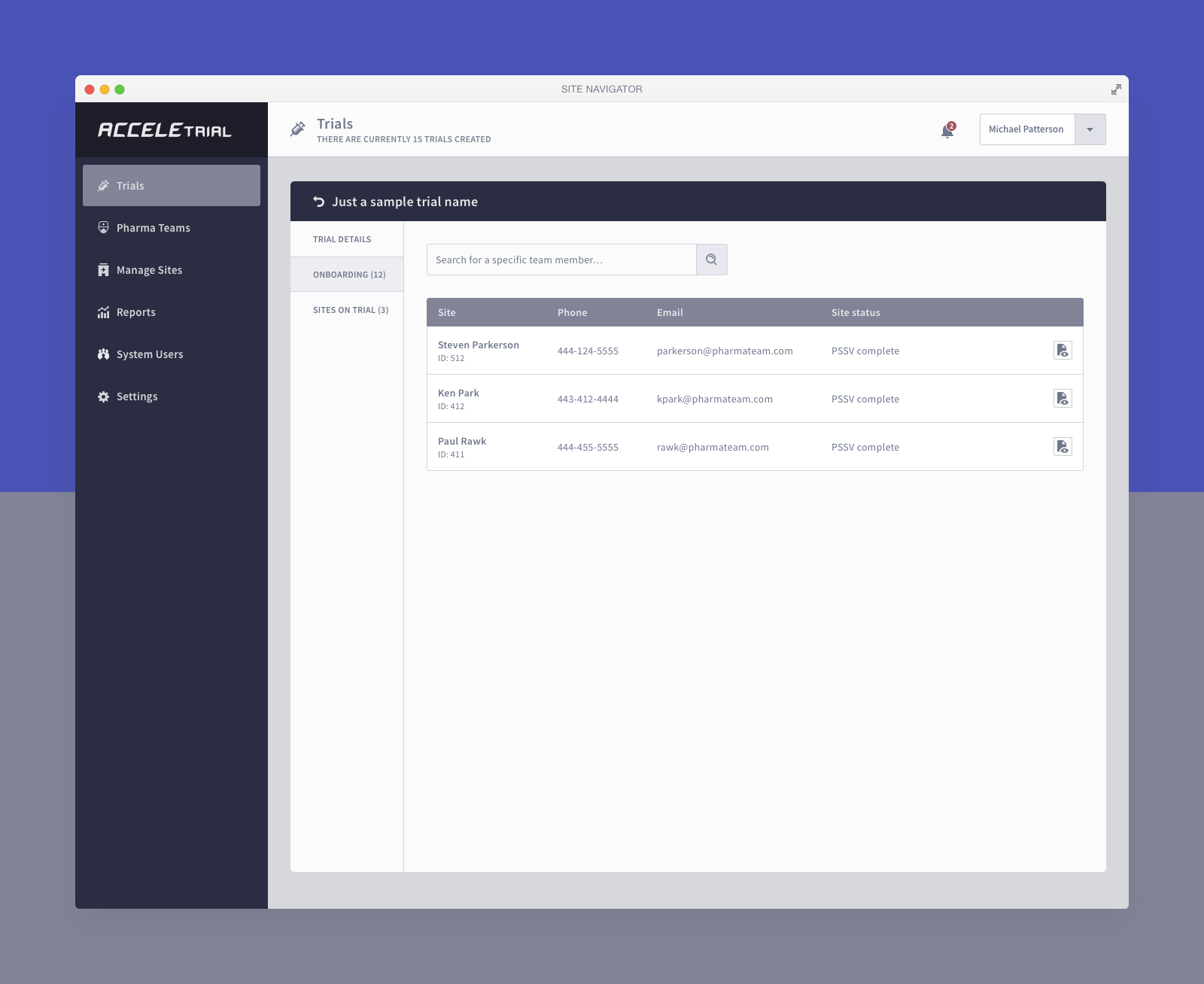
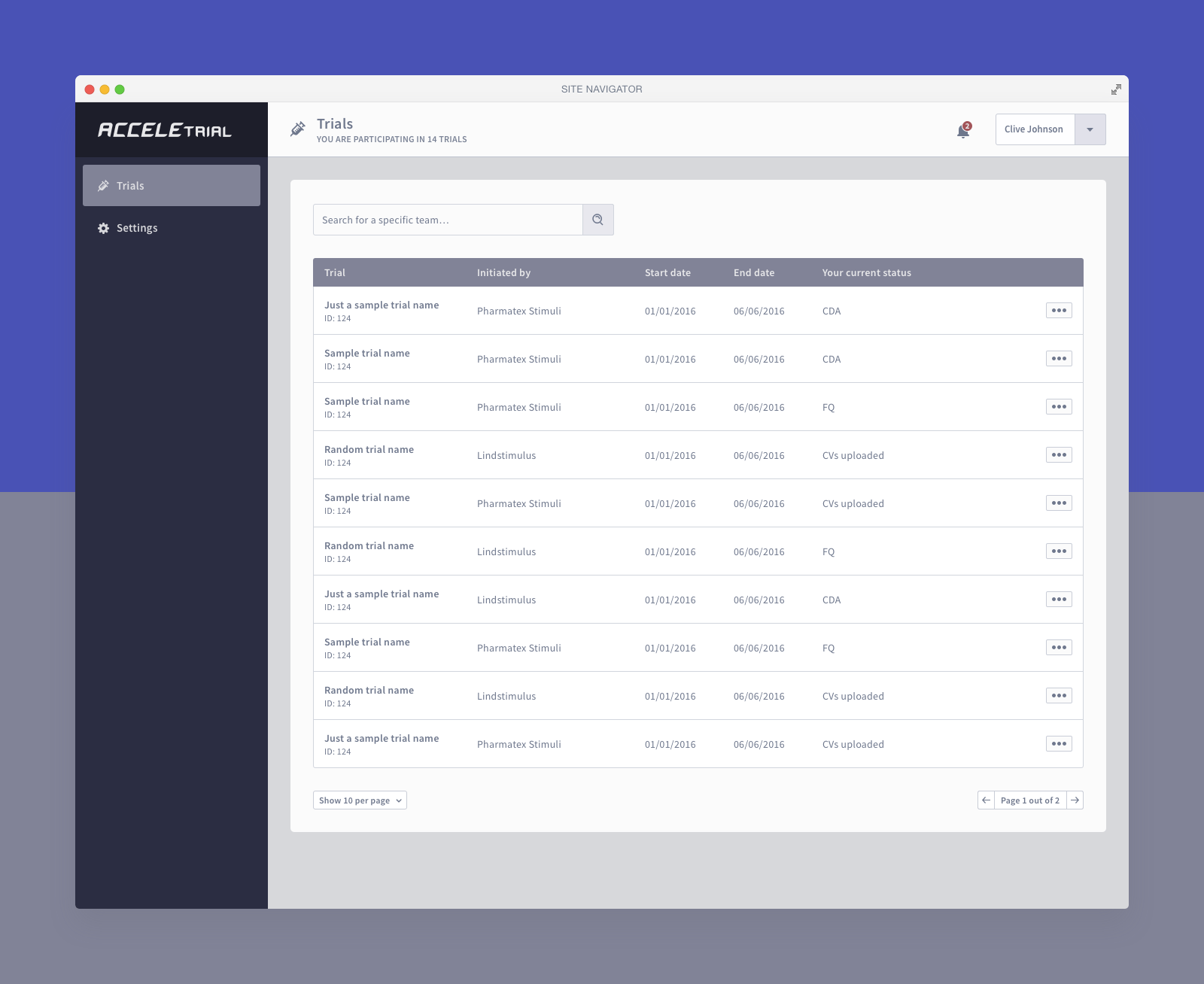
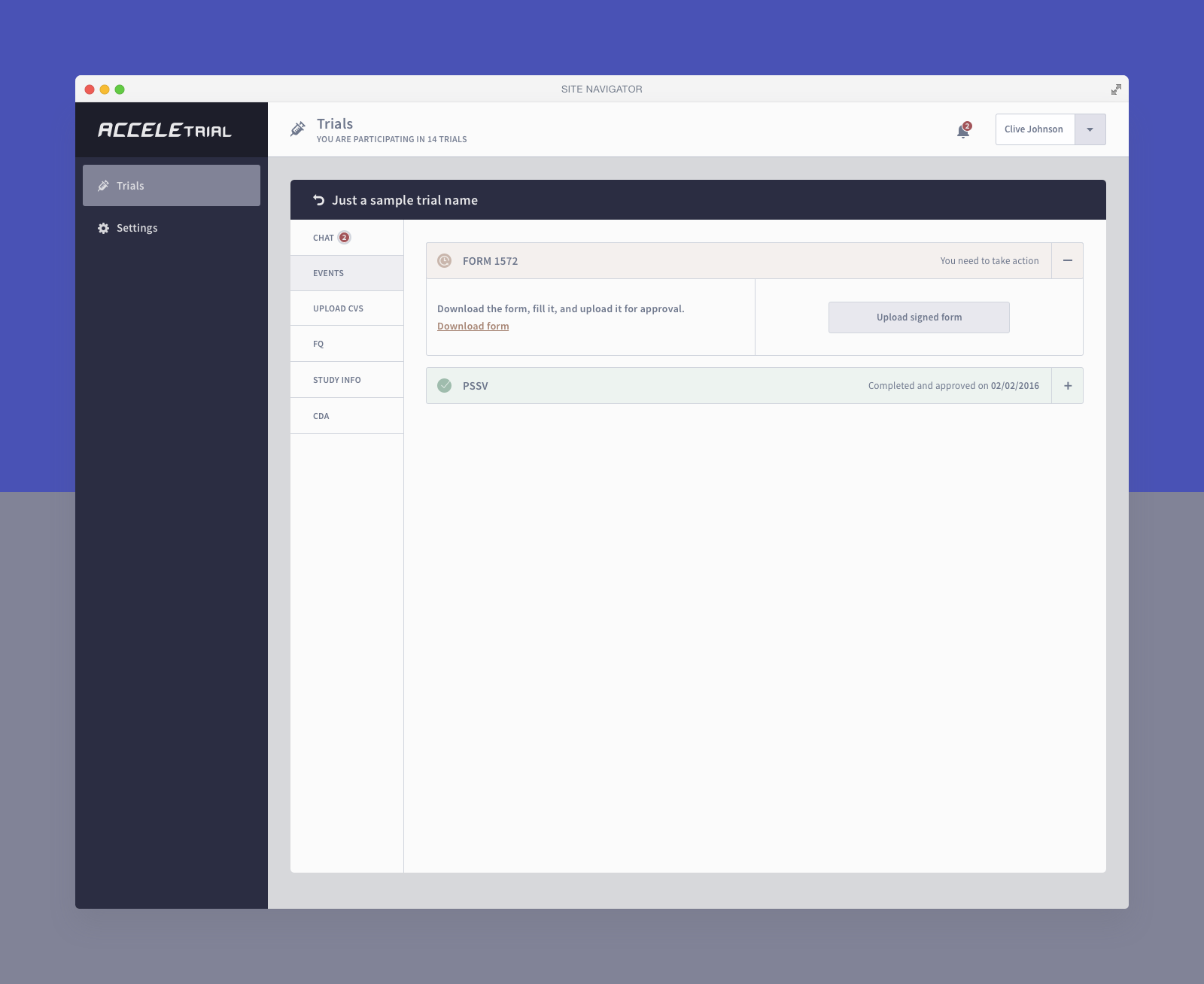
Below you can find a selected list of screens. Due to the sensitive nature of this project, this is all I can share.
FINAL
WORDS?
This was an interesting project to work on, especially since it was part of a closed/niche field. It was interesting diving into the clinical trials world, learning everything there is to learn about them, and about how things work.
Sure, the first few couple of weeks were overwhelming, but after that we just got the hang of it, and things went as smooth as butter.
Plus, I was lucky enough to be working with the founders, who were really helpful, and everytime I had questions, they were there to answear them. And this got translated into a great product.
©2021 ROBERT BERKI SENDS HIS REGARDS
SONG OF THE DAY: EVERYTHING2ME